The challenged patent covers the antiepileptic drug VIMPAT® (lacosamide). Since its introduction in 2009, VIMPAT® has generated more than $2.4 billion in net U.S. sales. Several generic manufacturers challenged VIMPAT®’s patent and alleged that it is obvious over the inventor’s prior publications.
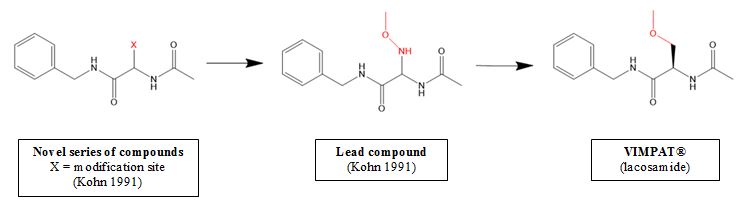
As illustrated in the diagram above, the central issue was whether changing the nitrogen (N) at the modification site (X) of the lead compound to a carbon (in other words, the process of creating VIMPAT®) would have been obvious. The petitioners showed that the modification was routinely performed in the pharmaceutical industry to reduce toxicity and improve activity and that replacing nitrogen with carbon, as would be necessary to arrive at VIMPAT®, was common in commercial pharmaceuticals.
While the petitioners seemed to have a tidy obviousness argument, they overlooked a key detail. The patent owner showed that, even though the proposed modification looks innocuous on paper, it would have produced compounds with substantially different three-dimensional structures that would have reduced anticonvulsant activity—not the desired effect of an antiepileptic drug. In fact, the inventor’s own publications showed reduced activity when changing the nitrogen in the lead compound. Thus, the change would not have been obvious, the patent owner argued.
The Board agreed with the patent owner. Weighing the specific teaching that the modification at issue was undesirable against the general knowledge that the modification was possible and common, the Board found the scales tipped in favor of the patent owner. In so doing, the Board reaffirmed the general rule that a motivation to modify compounds to improve their features simply may not overcome specific teachings that such a modification is unwanted.
Takeaways
Whether in the context of a multibillion-dollar dispute or not, the parties to an IPR should not lose track of the basics. In the context of pharmaceutical patents, petitioners should not neglect to show the motivation to modify the lead compound with the known modification. Showing mere knowledge of the modification is unlikely to lead to success, especially in the face of prior art teaching that the modification is not a good idea. Patent owners should be sure to find and point out how the claimed invention bucks the teachings of the prior art to do something that was not considered desirable.
Topics: Biotech

